15th March 2024 by Claire O'Donnell and O Wise Education OWiseCollaboration Support
Pembrolizumab, also known as Keytruda®, is a drug used to treat a variety of cancers1. In breast cancer, Keytruda is currently used to treat both early and secondary triple negative breast cancer (TNBC). Keytruda is a monoclonal antibody drug and is also a type of immunotherapy. This means that it works by boosting the body’s own immune system to attack cancer cells, rather than using more toxic treatments2. We wrote this blog in collaboration with O Wise.
What is Keytruda?
Keytruda is a type of protein called a monoclonal antibody, which is an antibody that are made in the lab. Antibodies are proteins that are made by the body to help fight infection3. They can bind to specific targets on the surface of cells, called antigens. These antigens recognise any foreign substances in the body, like viruses and bacteria. These are then flagged to the immune system and destroyed, preventing infection. The way that antibodies bind to specific target antigens is very specific. Scientists can design monoclonal antibodies to target an antigen of their choosing, enabling them to be used in the diagnosis and treatment of many diseases.
How does Keytruda work in breast cancer?
Keytruda is a monoclonal antibody that acts as an immunotherapy drug. It is a type of immunotherapy called an immune checkpoint inhibitor. Immune checkpoints are proteins on the cell surface, such as PD-L1, that help the immune system to recognise the body’s own cells. This happens through PD-L1 proteins binding to PD-1 receptors on T cells, which is a type of immune cell (figure 1). This stops the immune system from attacking these cells4.
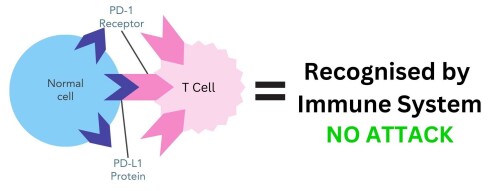
Figure 1. PD-L1 protein on normal cells binding to PD-1 receptor on T cells. The immune system recognises these cells as belonging to the body and does not attack.
In some cases, cancer cells can use checkpoint proteins to avoid being detected by the immune system. They can have PD-L1 proteins on their cell surface, which enables them to bind to PD-1 proteins on T cells and trick the body into thinking they are normal cells (figure 2).
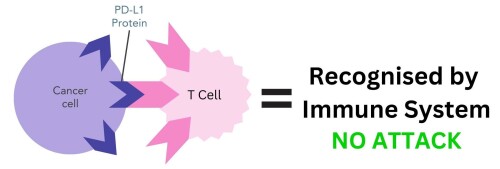
Figure 2. PD-L1 protein on cancer cells binding to PD-1 receptor on T cells. The immune system mistakenly recognises cancer cells as belonging to the body and does not attack.
Keytruda works by blocking the PD-1 receptors on T cells, preventing them from binding to the PD-L1 protein on the surface of breast cancer cells. This stops cancer cells from being able to hide from the immune system. T cells can now recognise, attack and destroy cancer cells4.
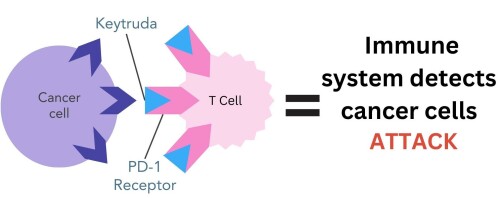
Figure 3. Pembrolizumab (Keytruda) drug binds to PD-1 receptor on T cells. This prevents the PD-L1 protein on cancer cells from binding to PD-1, enabling the immune system to recognise and destroy cancer cells.
Who is Keytruda for?
Keytruda may be a treatment option for patients with secondary, locally recurrent or inoperable TNBC5. Patients must not have been previously treated with chemotherapy for advanced disease. It is given in combination with paclitaxel (Taxol®) or nab-paclitaxel (Abraxane®) chemotherapy.
For Keytruda to be a treatment option, the breast cancer cells need to have a certain amount of the PD-L1 protein on the cell surface. To be eligible the tumour must have5:
A combined positive score (CPS) of 10 or more
Immune cell staining (IC) of less than 1%
Keytruda is an approved treatment option for secondary TNBC in the UK, US and EU5,7,8. It is also approved to treat high risk early TNBC.
What clinical evidence is there for Keytruda in treating breast cancer?
The KEYNOTE-335 trial tested Keytruda in 847 secondary TNBC patients that had PD-L1 on the cancer cell surface6. Patients were either treated with Keytruda combined with chemotherapy or chemotherapy combined with placebo. They tested these drugs in patients with varying amounts of the PD-L1 protein on the cancer cell surface.
High amount of PD-L1: In patients with a CPS of 10 or more, most lived 23.0 months longer on average when treated with Keytruda, compared to 16.1 months in the patients treated with placebo6.
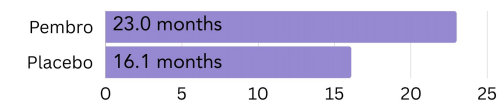
Figure 4. The amount of time patients with a high amount of PD-L1 on the surface (CPS >10) went on average without the cancer growing or spreading (progression free survival) on pembrolizumab (Keytruda) vs. placebo.
Low amount of PD-L1: In patients with less of the PD-L1 protein on the cell surface with a CPS of 1, those treated with Keytruda lived 17.6 months longer on average, compared to 16.0 months in the placebo group6.
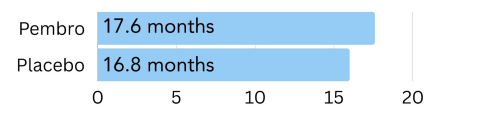
Figure 5. The amount of time patients with a low amount of PD-L1 on the surface (CPS of 1) went on average without the cancer growing or spreading (progression free survival) on pembrolizumab (Keytruda) vs. placebo6.
Unknown amount of PD-L1: In patients with an unknown CPS, those treated with Keytruda lived 17.2 months longer on average, compared to 15.5 months for those treated with placebo.
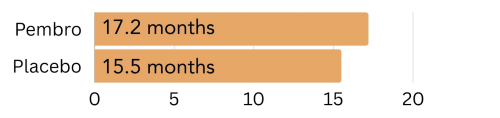
Figure 6. The amount of time patients with an unknown amount of PD-L1 on the surface went on average without the cancer growing or spreading (progression free survival) on pembrolizumab (Keytruda) vs. placebo.
These results show patients that have the biggest benefit from treatment with Keytruda are those with a high amount of the PD-L1 protein on their surface (CPS of 10 or more)6.
What side effects can Keytruda cause?
Serious side effects to look out for:
As Keytruda works with your immune system, there is a chance that it can cause the immune system to attack non-cancer cells and normal organs in your body1. This can occur at any time during or after treatment, and under some circumstances, you may have to stop Keytruda treatment. It is important to be aware of the signs and report them to your care team1:
Lung problems – cough, shortness of breath, chest pain
Intestinal problems – diarrhoea, black, tarry, sticky stools, blood or mucous in stool, stomach pain or tenderness
Liver problems – jaundice, nausea or vomiting, pain in the abdomen, dark urine, bleeding or bruising easily
Hormone gland problems – persistent headaches, eye problems, rapid heartbeat, sweating, extreme tiredness, weight gain or loss, appetite changes, hair loss, feeling cold, very thirsty, constipation, deepening of the voice, dizziness, fainting, changes in mood or behaviour
Kidney problems - decrease in urine, blood in urine, swelling of the ankles, loss of appetite
Skin problems – Rash, itching, skin blistering or peeling, painful sores or ulcers in the mouth, nose, throat or genital area, fever, swollen lymph nodes
Organ and tissue problems – chest pain, irregular heartbeat, shortness of breath, swelling of the ankles, confusion, sleepiness, memory problems, changes in mood or behaviour, stiff neck, balance problems, tingling or numbness of the arms or legs, eye problems, muscle pain, weakness or cramps, low red blood cell count, bruising
Infusion reactions – chills, shaking, itching, rash, flushing, shortness of breath, wheezing, dizziness, fever, back pain
Common side effects of Keytruda
There are other common side effects that occur in response to Keytruda treatment, including1:
Feeling tired
Pain in the bones, muscle joints, stomach
Nausea
Rash
Diarrhoea
Fever
Cough
Decreased appetite
Shortness of breath
Itching
Constipation
Low levels of the thyroid hormone
In any case where these symptoms become unmanageable, seek advice from your care team or general practitioner.
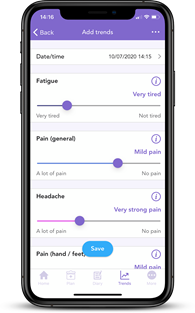
Have you tried tracking your side effects on OWise? With our easy-to-use sliders, you can track over 30 different side effects. This makes it simple to monitor and track key side effects you need to look out for. You can even share your trends directly with your care team and loved ones.

What is your secondary breast cancer diagnosis background?
My initial diagnosis had been oesteogen receptor-positive (ER+) breast cancer and I was on abemaciclib (Verzenio®) and Letrozole (Femara®) treatment. I had progression to lymph nodes to the right axilla and abdomen while I was on these treatment lines. I had also had my ovaries removed so very little oestrogen was entering my body. I know we can become resistant to drugs but had also heard of people changing subtype so wanted to have this checked out. I spoke to my oncologist who said we could ask my breast surgeon to biopsy the nodes in the axilla which did indeed turn out to be triple negative.
How did this lead to you starting Keytruda treatment?
When the biopsy came back they sent the samples to Birmingham to be tested for the PD-L1 protein. Meanwhile we popped to Athens on holiday, knowing I would have to start new treatment when I got back. Fortunately, I tested positive for PD-L1 and started on Keytruda every 6 weeks alongside Abraxane chemotherapy every week for 3 weeks.
What side effects did you experience on Keytruda?
I tolerated the treatment pretty well side effects wise. A bit of tiredness and hair thinning which led to me having a little pixie cut from my chin length bob as my ears were poking through and I didn't like it. I didn't lose my hair but did lose my eyelashes and brows.
Have you had any scans while on Keytruda?
My first scan showed a mixed bag but by the next scan I had been diagnosed with adrenal insufficiency after an admission to the Cancer Assessment Unit and had some progression so I am now on sacituzuamb govitecan (Trodelvy®). I know the Abraxane/ Keytruda combination had been successful, and I am grateful for the 6 months I had on it.

What is your secondary diagnosis background?
Back in 2014 I was diagnosed with hepatocellular cancer (HCC), a type of liver cancer. I had a 9x11cm tumour and had the whole of the left lobe removed. Six months later it returned in the liver and lungs. Liver Cancer has poor outcomes and limited treatments. By 2016 I was struggling with the liver mets in my lungs and pestered for immunotherapy. I was lucky to be accepted on a phase 2 clinical trial for pembrolizumab (Keytruda®).
What was your experience on Keytruda?
Immunotherapy at the time was only approved for 2 years of use. Although at the end of the trial there was still evidence of tumours, they were much reduced in size and some were described as necrotic/scar tissue. Because of the way Immunotherapy works, the immune system continues to respond even after stopping the actual drug. Five years after the trial, it's now reported as a complete response and both my liver and lungs are clear from stage 4 HCC cancer.
The key to pembrolizumab is the amount of the PD-L1 protein on the cell surface. It is now recognised that there is a greater success with a higher amount of PD-L1. To detect your level will require a biopsy. Although pembrolizumab cleared my stage 4 liver cancer, it had no effect on a small breast tumour. At that time PD-L1 testing was not being done. I have since learnt that the breast tumour was TNBC and my PD-L1 had a combined positive score (CPS) < 10, which explained why it did not respond. Although the addition of pembrolizumab to chemo is an improvement on previous treatments, it is still not a huge improvement in the overall survival times that we in the metastatic community would like.
Keytruda is a promising immunotherapy that is effective in treating secondary TNBC. It has impressive clinical data in patients with a high amount of the PD-L1 protein on the cancer cell surface. As with any medical intervention, it is important to have effective communication with your care team ensures optimal management and support if you are undergoing this innovative treatment.
For more on this series check out 'Secondary breast cancer: pathology, signs and diagnosis' and the Make2ndsCountwebsite for more on the emotional impact of secondary breast cancer.
At OWise, we want to make sure you are kept informed so make sure to follow our Instagram and Facebook for any updates. Any questions? Get in touch!
References
Merck & Co. KEYTRUDA® (pembrolizumab). wwwkeytrudacom. [accessed 2023 Jul 19]. https://www.keytruda.com
Shimu AS, Wei H, Li Q, Zheng X, Li B. 2022 Sep 15. The new progress in cancer immunotherapy. Clinical and Experimental Medicine. doi: https://doi.org/10.1007/s10238-022-00887-0
Vachani C. 2023 Feb 6. Pembrolizumab (Keytruda®) | OncoLink. wwwoncolinkorg. [accessed 2023 Jul 19]. https://www.oncolink.org/cancer-treatment/oncolink-rx/pembrolizumab-keytruda-r#:~:text=About%3A%20Pembrolizumab%20(Keytruda.
Breast cancer organisation. 2023 May 25. Learn How the Immunotherapy Medicine Keytruda Treats Breast Cancer. wwwbreastcancerorg. [accessed 2023 Jul 19]. https://www.breastcancer.org/treatment/immunotherapy/keytruda.
NICE. 2022. Final draft guidance adds further treatment option for triple-negative breast cancer | News | News. NICE. [accessed 2023 Jul 20]. https://www.nice.org.uk/news/article/nice-final-draft-guidance-adds-further-treatment-option-for-triple-negative-breast-cancer.
Cortes J, Rugo HS, Cescon DW, Im S-A, Yusof MM, Gallardo C, Lipatov O, Barrios CH, Perez-Garcia J, Iwata H, et al. 2022. Pembrolizumab plus Chemotherapy in Advanced Triple-Negative Breast Cancer. New England Journal of Medicine. 387(3):217–226. doi: https://doi.org/10.1056/nejmoa2202809
FDA. 2020. FDA grants accelerated approval to pembrolizumab for locally recurrent unresectable or metastatic triple negative breast cancer. FDA. [accessed July 2023 Jul 20]. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-locally-recurrent-unresectable-or-metastatic-triple
EMA. 2021. Keytruda | European Medicines Agency. EMA. [accessed July 2023 Jul 20]. https://www.ema.europa.eu/en/documents/product-information/keytruda-epar-product-information_en.pdf