Triple Negative Breast Cancer Mutation Profiling Study
In 2019 Make 2nds Count awarded it's first research grant to Dr Olga Oikonomidou, Consultant Medical Oncologist and Leader of The University of Edinburgh Breast Cancer Translational Research Group at the Edinburgh Cancer Research Centre.

This grant was used to fund a 3-year post-doctoral researcher post, held by Dr Fiona Semple within Dr Oikonomidou's team.
The aim of this project was to investigate how mutations (i.e., genetic changes) within Triple Negative Breast Cancer (TNBC) tumours change and evolve over time, from initial diagnosis to recurrence.
The project also investigated the use of cutting-edge, but cost-effective laboratory methods so that this research could be conducted at a larger scale in the future and thereby benefit a higher number of patients.
Triple Negative Breast Cancer
TNBC accounts for ~15% of all breast cancer cases and around 8,000 women are diagnosed with TNBC in the UK each year. TNBC is harder to treat than other breast cancer types, has a higher risk of spreading and disproportionately affects women under 40 and those from black backgrounds. TNBC tumours lack receptors for the hormones oestrogen and progesterone and also lack Human Epidermal Growth Factor Receptor 2 (HER2). Cancer treatments (such as hormonal therapy and anti-HER2 therapies) can target these receptors, but can't be used in the treatment of TNBC.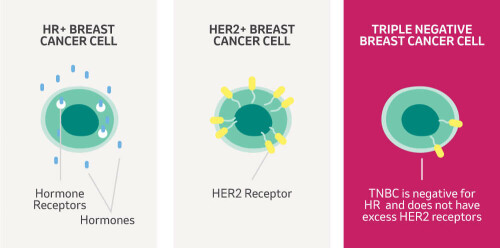 Most TNBC patients receive chemotherapy before surgery to shrink their tumour. This is known as neoadjuvant chemotherapy (NACT).
Most TNBC patients receive chemotherapy before surgery to shrink their tumour. This is known as neoadjuvant chemotherapy (NACT).
More research needs to be conducted to better understand how the genetic make-up of TNBC evolves/changes in response to NACT, as this could influence patient treatment outcomes and survival.
Compared to normal samples, the genetic mutations within a TNBC tumour can be on a small scale where only single letters of DNA are altered (known as Single Nucleotide Variants or SNVs) or a large scale (known as Structural Variants, such as deletion and duplications of larger regions of chromosomes).
This project researched how these small and larger scale mutations within the TNBC tumour changed through time and provided a better understanding of which of these changes might be associated with the tumour going into remission or recurrence.
Study Design & Methods
Samples were collected from patients at the following time points (Figure 1):
- Blood samples were collected pre-treatment as non-tumour reference samples that tumour samples could be compared too.
- The diagnostic breast tumour biopsy sample was collected (before any treatment).
- After surgery and after NACT a further breast biopsy was collected.
- If the tumour spread, samples were collected from sites of recurrence.
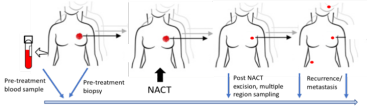
Figure 1: Study Sampling
The collected samples were subjected to two types of laboratory techniques, so that both the small and larger scale mutations within the TNBC tumour could be investigated. Whole Exome Sequencing was used for the identification of SNVs and CUTSeq was used for the identification of structural variants.
CUTSeq is a cutting edge laboratory method that is more cost effective than previous methods because it uses less reagents by enabling more samples to be analysed at the same time.
Preliminary Study Findings
Dr Fiona Semple presented some preliminary study findings at the Shining a Light on Breast Cancer event in March 2023 hosted by the Institute of Genetics and Cancer at the University of Edinburgh, which can be viewed here:
Preliminary analysis has shown, as expected, that initial breast tumour biopsy samples collected before any treatment had taken place had high levels of large scale genetic mutations (such as chromosomal duplications and deletions).
 Interestingly, breast tumour samples collected after surgery and after NACT have shown the identified large scale genetic mutations in the pre-treatment tumour have changed through time. This suggests the chemotherapy treatment before surgery might have altered how these genetic mutations change and evolve through time.
Interestingly, breast tumour samples collected after surgery and after NACT have shown the identified large scale genetic mutations in the pre-treatment tumour have changed through time. This suggests the chemotherapy treatment before surgery might have altered how these genetic mutations change and evolve through time.
Some of these changes that occur might then be associated with different patient treatment outcomes, such as with the tumour staying in remission after surgery, or returning and spreading into secondary breast cancer sites.
Analysis of secondary breast cancer tumours that did spread through the body has shown that although some similarities with the original tumour sample still exist, further structural changes can be identified.
By researching the changes that are associated with different patient treatment outcomes, a further understanding of what changes are associated with tumour recurrence and secondary spread can be developed. There is then the possibility that these identified genetic changes could become targets for drug development in the future.
The full findings of this study are being prepared for publication in an academic journal and will be shared on the Make 2nds Count website after publication.